Epilepsy
-
Increased levels of small extracellular vesicles in the blood of patients with depression, epilepsy and epilepsy with depression
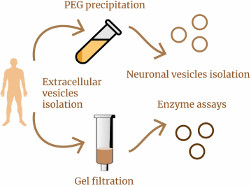
A recently published article by researchers from the IHNA&NPh RAS and the Moscow Research and Clinical Center for Neuropsychiatry raises new and interesting questions in the study of small extracellular vesicles (sEVs) in human blood. Previously, the team, using four different methods, had shown significant increase in the concentration of sEVs in the blood of patients with depression compared to healthy volunteers, and the followed work was intended to clarify the source of these “extra” vesicles in depression. In addition, the authors compared sEVs content in the blood of patients with a wide range of pathologies, including epilepsy, epilepsy with depression, bipolar affective disorder with a current depressive episode, and psychogenic nonepileptic seizures with depression. Small EVs were isolated from patient serum using gel filtration or polyethylene glycol (PEG) precipitation, and both methods showed very similar results. It turned out that in patients with depression, epilepsy, or epilepsy with depression, the total level of mBB in the blood is increased by up to two times compared to healthy volunteers. The authors of the work were able to go further and isolate the fraction of sEVs of neuronal origin (in the blood this is approximately every one hundred and fiftieth vesicle) in these patients, but no difference in the concentration of neuronal sEVs was found between any groups. The question of where the “extra” sEVs in the patients’ blood came from remains open. The authors suggest that the source of these sEVs are immune cells. However, the authors did discover at least one new finding that sheds light on the biogenesis of sEVs. It turned out that sEVs in the serum of both patients and healthy volunteers contain various lysosomal enzymes - and this is a hint that the contents of sEVs may reflect the state of the intracellular endolysosomal system.
-
Донор метильных групп L-метионин – новый антидепрессант с анксиолитическим и антиэпилептическим действием

Терапия депрессивных расстройств, коморбидных эпилепсии, является серьезной проблемой, так как традиционные антидепрессанты (используемые в клинике) либо оказываются неэффективными, либо вызывают усиление сопутствующей эпилепсии. Поэтому экспериментальные исследования новых препаратов с принципиально новыми механизмами действия, способных оказать лечебный эффект на обе коморбидные патологии, имеет не только теоретическое, но и большое практическое значение для трансляционной медицины. Сотрудники ИВНД и НФ РАН впервые показали, что донор метильных групп L-метионин уменьшает уровень тревожности и депрессии, а также подавляет симптомы сопутствующей absence-эпилепсии у крыс линии WAG/Rij, в отличие от традиционного антидепрессанта имипрамина, который усиливает абсанс-эпилепсию и может вызвать абсансный статус. Терапевтический эффект L-метионина сопровождался повышением содержания дофамина, норадреналина и их метаболитов в структурах мозга, вовлеченных в патогенез депрессии и абсанс-эпилепсии.
Ключевые слова: депрессия, эпилепсия, антидепрессанты, нейрохимия мозга Статья опубликована в августе 2023 года в журнале International Journal of Molecular Sciences:
Статья опубликована в августе 2023 года в журнале International Journal of Molecular Sciences: -
Нейро-когнитивные нарушения, сопутствующие абсанс-эпилепсии
7 января 2024 года в журнале Биомедицина (Biomedicines) опубликован обзор д.б.н. Е.Ю.Ситниковой о проблеме поведенческих и нейро-когнитивных нарушений у крыс с генетической склонностью к абсансной эпилепсии. Абсансная эпилепсия – это неконвульсивная эпилепсия, которая проявляется как внезапное и резкое снижение уровня сознания. Данная болезнь вызвана нарушениями в таламо-кортикальной системе и сопровождается расстройствами в нейро-когнитивной сфере. Абсанс-подобные приступы наблюдаются у многих линий лабораторных крыс-альбиносов (например, Wistar, Sprague–Dawley, and Long Evans). У взрослых крыс Wistar часто наблюдают спонтанные абсанс-подобные приступы, поэтому следует соблюдать осторожность при использовании крыс Wistar в качестве контроля. Специально выведенные крысы GAERS (Genetic Absence Epilepsy Rats from Strasbourg) и и WAG/Rij (Wistar Albino Rats from Rijswijk) являются генетическими моделями абсанс-эпилепсии, и у них отмечают нарушения поведения и когнитивных функций, сходные с таковыми у пациентов с абсанс-эпилепсией. У крыс WAG/Rij дефицит управляющих функций и ухудшение памяти связаны с тяжестью эпилепсии. В обзоре обсуждается депрессивное и тревожно-подобное поведение у крыс GAERS и WAG/Rij, половые различия сопутствующих когнитивных нарушений, а также высокая эмоциональность у генетически предрасположенных крыс. Для понимания механизмов нарушения нейро-когнитивных функций при абсанс-эпилепсии автор предлагает использовать концепцию «когнитивный таламус» (the cognitive thalamus).
Ключевые слова: генетические модели животных; спонтанная абсансная эпилепсия; крысы, не подвергавшиеся фармакотерапии; обучение, мотивированное страхом; тревожность; депрессивно-подобные симптомы; когнитивный таламус.
-
Neurocognitive comorbidities of absence epilepsy
On January 7, 2024, Dr. Evgenia Sitnikova's review on behavioral and neuro-cognitive comorbidities in rats with a genetic predisposition to absence epilepsy was published in the journal Biomedicines. Absence epilepsy is a non-convulsive type of epilepsy characterized by a sudden decrease in consciousness. Absence epilepsy is a disorder of the thalamo-cortical system, and it is accompanied by neurocognitive comorbidities. Absence-like seizures have been observed in many strains of albino laboratory rats, including Wistar, Sprague–Dawley, and Long Evans. Spontaneous absence-like seizures are common in adult Wistar rats, so caution should be exercised when using Wistar rats as controls. The specially bred GAERS (Genetic Absence Epilepsy Rats from Strasbourg) and WAG/Rij (Wistar Albino Rats from Rijswijk) rats are genetic models of absence epilepsy, which show behavioral and cognitive impairments similar to those of patients with absence epilepsy. In WAG/Rij rats, deficit of executive function and impairment of memory are associated with epilepsy severity. This review discusses depressive and anxiety-like behavior in GAERS and WAG/Rij rats, sex differences in concomitant cognitive impairment, and high emotionality in genetically predisposed rats. In order to better comprehend the causes of neurocognitive dysfunction in absence epilepsy, the author suggests using the concept of the "cognitive thalamus".
Keywords: genetic animal models; spontaneous absence epilepsy; drug-naive rats; fear-motivated learning; anxiety-like symptoms; depression-like symptoms; cognitive thalamus
-
Функции астроцитарной нейроглии в развитии эпилепсии
Эпилепсия – это хроническое заболевание, характеризующееся не только нарушениями нейронной активности, но и существенными изменениями глиальных клеток. Ведущий научный сотрудник нашего института д.б.н. Е,Ю.Ситникова совместно с коллегами из Стамбульского университета и университета Аджибадема Мехмета Али Айдинлара (Стамбул, Турция) опубликовали обзор, где систематизировали нарушения функций астроцитов в развитии эпилепсии, включая эпилептогенез (генерация судорожной активности) и иктогенез (генерализция судорожной активности). Отдельное внимание уделяется новым стратегиям таргетной терапии эпилепсии, направленным на регуляцию функций астроцитарной нейроглии: (1) избирательное воздействие на нейроглиальные молекулярные механизмы транспорта глутамата; (2) модуляция тонического высвобождения ГАМК из астроцитов; (3) модуляция глиотрансмиссии; (4) воздействие на астроцитарную систему Kir4.1-BDNF; (5) модуляция астроцитарной Na+/K+/АТФазная активности; (6) коррекция гипо- или гиперметилирования генов-кандидатов в ДНК астроцитов; (7) воздействие на регуляторы астроцитарных щелевых соединений; (8) воздействие на астроцитарную аденозинкиназу (основной фермент, метаболизирующий аденозин); и (9) воздействие на нейровоспаление с вовлечением микроглии и астроцитов.
Подробности изложены в этой статье [1], опубликованной в июле 2023 года в журнале Frontiers in Molecular Neuroscience.
-
Диета матери подавляет проявление эпилепсии и депрессии у потомства

Диета матери во время перинатального периода является важнейшим ранним средовым фактором, способным за счет эпигенетических модификаций вызвать изменение экспрессии генов и, как следствие, фенотип потомства. Сотрудники института ВНД и НФ РАН и института молекулярной генетики РАН впервые показали, что усиление метилирования ДНК на ранних стадиях онтогенеза с помощью диеты матери, обогащенной метильными добавками (МОД), подавляет проявление генетической абсанс-эпилепсии и коморбидной депрессии у потомства крыс линии WAG/Rij. Благоприятный фенотипический эффект МОД матери сопровождался усилением экспрессии патогенетически значимых генов – гена ионного канала hcn1 и гена ДНК метилтрансферазы 1 (dnmt1) в соматосенсорной коре и гиппокампе, а также генов hcn1, dnmt1 и тирозингидроксилазы (th) в прилежащем ядре. Терапевтический эффект МОД матери был аналогичен эффекту этосуксимида - препарата первого выбора для лечения абсанс-эпилепсии. Предполагается, что МОД матери может служить новой превентивной терапевтической стратегией для эпигенетической коррекции абсанс-эпилепсии и коморбидной депрессии у потомства.
Ключевые слова: эпилепсия, депрессия, молекулярная генетика
Статья опубликована в январе 2023 года в журнале Diagnostics:
https://doi.org/10.3390/diagnostics13030398 -
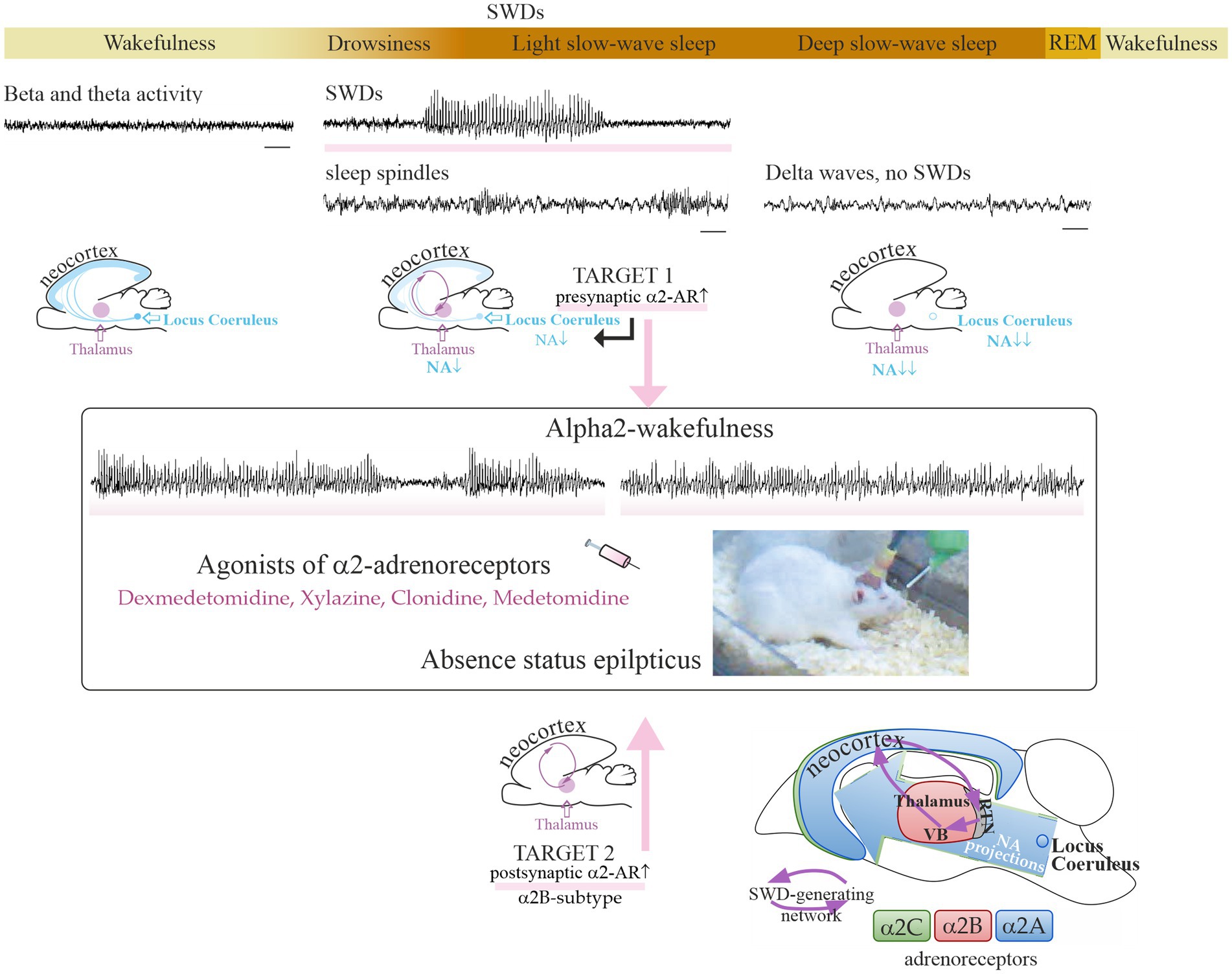
The brain's adrenergic control of sleep and epilepsy
There are two main types of adrenergic receptors (alpha and beta) with several subtypes in the human and animal brain. Alpha2-adrenergic receptors are of particular interest, because they are involved in the modulation of sleep and absence epilepsy. In 2023, a group of young scientists led by a senior researcher, Dr. Evgenia Sitnikova, have studied the adrenergic mechanisms of sleep and epilepsy. The results of their research were published in the International Journal of Molecular Science and Frontiers in Neurology [1, 2, 3]. Therapeutic doses of alpha2 adrenergic agonists (such as dexmedetomidine) are known to cause sedation and drug-induced sleep in humans and animals. These drugs in low doses were found to induce spike-wave activity in the electroencephalogram, i.e. manifestation of absence epilepsy, in genetically prone rats (WAG/Rij) [1, 2, 3]. А single (intraperitoneal) injection of dexmedetomidine at the dose of 0.005 mg/kg increased absence epilepsy in WAG/Rij rats (i.e., genetic model of absence epilepsy) up to status epilepticus in subjects with severe absence epilepsy, but did not cause de novo absence epilepsy in asymptomatic rats [1, 2]. Dexmedetomidine has been regularly used in clinical practice for decades, and low doses of this drug may help in the diagnosis of latent forms of absence epilepsy during EEG examination. Dexmedetomidine, along with other central alpha2-adrenergic agonists, could be a pharmacological tool for differential diagnosis. The high risk of provoking absence status should be taken into account when using alpha2-adrenergic receptor agonists in patients with absence epilepsy or with a genetic predisposition to this disease [1].
A new concept of targeted pharmacotherapy of absence epilepsy using alpha2B-adrenergic receptor antagonists was proposed based on our experimental and literature data [1, 3].
- Sitnikova E.Adrenergic mechanisms of absence status epilepticusFront. Neurol. 2023 14: 1298310. DOI: 10.3389/fneur.2023.1298310
- Sitnikova E., Pupikina M.,Rutskova E.Alpha2 Adrenergic Modulation of Spike-Wave Epilepsy: Experimental Study of Pro-Epileptic and Sedative Effects of Dexmedetomidine. International Journal of Molecular Sciences. 2023. 24(11): 9445. DOI: 10.3390/ijms24119445 .
- Sitnikova E., Rutskova E., Smirnov K.Alpha2-Adrenergic Receptors as a Pharmacological Target for Spike-Wave Epilepsy. International Journal of Molecular Sciences. 2023. 24(2):1477. DOI: 10.3390/ijms24021477.
The illustration [3]licensed under CC BY 4.0. © 2023 authors
-
A Translational Study on Acute Traumatic Brain Injury: High Incidence of Epileptiform Activity on Human and Rat Electrocorticograms and Histological Correlates in Rats
In humans, early pathological activity on invasive electrocorticograms (ECoGs) and its putative association with pathomorphology in the early period of traumatic brain injury (TBI) remains obscure. Our scientists from the Laboratory of Functional Biochemistry of Nervous System assessed pathological activity on scalp electroencephalograms (EEGs) and ECoGs in patients with acute TBI, early electrophysiological changes after lateral fluid percussion brain injury (FPI), and electrophysiological correlates of hippocampal damage (microgliosis and neuronal loss), a week after TBI in rats. They revealed that epileptiform activity on ECoGs was evident in 86% of patients during the acute period of TBI, ECoGs being more sensitive to epileptiform and periodic discharges. A “brush-like” ECoG pattern superimposed over rhythmic delta activity and periodic discharge was described for the first time in acute TBI. In rats, FPI increased high-amplitude spike incidence in the neocortex and, most expressed, in the ipsilateral hippocampus, induced hippocampal microgliosis and neuronal loss, ipsilateral dentate gyrus being most vulnerable, a week after TBI. Epileptiform spike incidence correlated with microglial cell density and neuronal loss in the ipsilateral hippocampus. Based on these results, our employees concluded that epileptiform activity is frequent in the acute period of TBI period and is associated with distant hippocampal damage on a microscopic level. This damage is probably involved in late consequences of TBI. The FPI model is suitable for exploring pathogenetic mechanisms of post-traumatic disorders